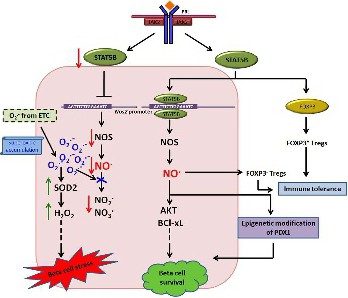
Fig. 6. Cartoon depicting the proposed role of STAT5B in regulation of oxidative stress in MIN6 β-cell line. The boxed area illustrates our hypothesis on regulation of nitric oxide by STAT5B. Binding of STAT5B to Nos2 promoter results in transcriptional activation of NOS2 and increased nitric oxide synthesis. This can augment prosurvival pathways by regulating AKT and BCL-xL expression and enables β-cell survival. This is in addition to the existing knowledge that STAT5B-regulated FOXP3+ T-regulatory cells (Tregs) and NO.-regulated FOXP3- Tregs promote immune tolerance, and nitric oxide can cause epigenetic modification of PDX1 enabling β-cell differentiation. When there is a dearth of STAT5B, Nos2 activation is decreased with a diminished production of nitric oxide and its end products. At the same time, more superoxide anions from electron transport chain are available for SOD to be converted to H2O2. With no corresponding increase in H2O2 metabolizing enzymes, the surge in H2O2 results in β-cell stress.